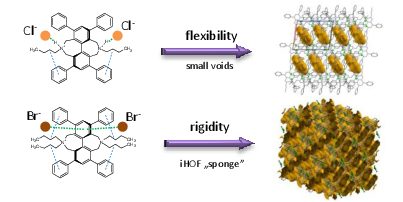
Dániel Vajk Horváth, Tamás Holczbauer,* Laura Bereczki, Roberta Palkó, Nóra Veronika May, Tibor Soós and Petra Bombicz CrystEngComm (2018), accepted for publication The polymorphism of a porous, non-covalently bonded ionic organic framework is reported. The framework is constructed by hydrogen bonding and anion⋯π interactions. In a solvatomorphic lattice, pyridine takes part in the framework formation. The role of molecular rigidity in framework construction is proven by analogous non-porous crystals, where polymorphism also appears. Full text