Basics of “crystal engineering”: supramolecular interactions, polymorphy, temperature and pressure
Basics of “crystal engineering”: supramolecular interactions, polymorphy, temperature and pressure
Our research group has a long tradition in both supramolecular chemistry and polymorphy studies. Isostruturality indices were introduced, morphotropy was described, and the first examples of morphotropy induced by supramolecular effects were revealed by our group. Combining all these knowledge we show how to fine tune structural properties by gradual chemical change. We demonstrate how the well balanced spatial and electrostatic forces play role in the arrangement of packing motifs in the crystals. Our research interest is extended on supramolecular interactions of one and multicomponent systems under ambient and non-ambient conditions.
Performing crystal engineering, e.g. fine tuning of crystal architecture requires the recognition, understanding and application of supramolecular interactions, crystallographic and in case of occurrence, non-crystallographic symmetries. The diversity and scope of intermolecular interactions in the solid state are investigated. The main emphasis is made on the relationship of secondary interactions and polymorphy. An improved understanding of supramolecular interactions at ambient and extreme conditions is in our interest in context of polymorphism, and the assembly of molecular crystals. We focus our work on crystal engineering, supramolecular chemistry of one and more component systems of biologically relevant organic molecules, organometallic compounds and metal coordination complexes. The focal points: how far the molecular conformation and the packing arrangement can be preserved in respond to chemical changes, e.g. sterical and electrostatical aspects.
Development of crystallization processes for creating solid state molcular associations.
Our goal is to obtain and determine the structures of new polymorphic or isostructural crystal forms of small organic molekules, particularly active pharmaceutical ingredients (APIs) by single crystal X-ray diffraction and several thermal analytic methods. It includes the development of laboratory crystallization processes to grow single crystals of one- or two-component systems, clathrates, inclusion complexes and co-crystals. Physico-chemical properties can be gradualy modified e.g. solidity that is related to the formulation, solubility that is connected to bioavailability in human body and stability that infulences the durability.
Structural investigation of bioactive organic molecules and their metal complexes
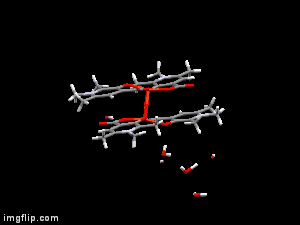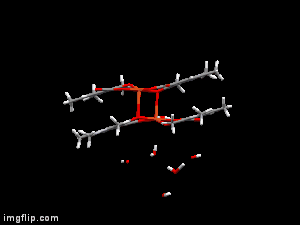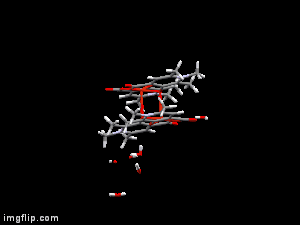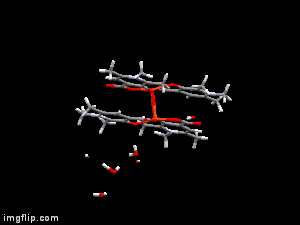
In diverse fields of clinical practices, metal complexes of small biomolecule are frequently used as bioactive compounds eg. drugs, imaging agents, or chelators. X-ray diffraction method is one of the most powerful technique for the investigation of single crystals of these organic compounds and their complexes. Though crystallographic method can offer detailed and accurate data on the structure of metal-bioligand complexes, it is limited only for solid states. In order to establish any structure-stability-activity relationships for these bioactive compounds the knowledge of the speciation, and the most plausible chemical forms, in aqueous solution is mandatory. Electron paramagnetic resoncance (EPR) spectroscopy is able to detect paramagnetic metal complexes in solution equilibrium systems. Such a structural comparison obtained at different phases can disclose interesting features of the intramolecular and intermolecular interactions of the complexes, and reveal possible structural transformations which can be crucial both for their biological functions and pharmaceutical formulations.