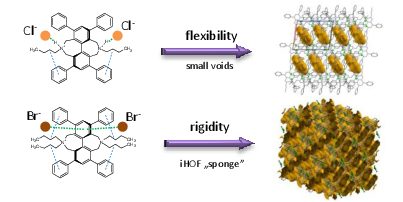
P. Bombicz, IUCrJ (2024) 11, 1-2 The phenomenon and definition of isostructurality are discussed. It deserves reconsideration regarding the aspects of symmetry, measure of similarity and formation of supramolecular interactions. Investigation of isostructurality leads to a deeper understanding of closepackingprinciplesandcontributestotheabilityofcrystalengineering.Agiven packingmotifmay tolerate small molecular changes within a limit. Slight alterationsofacrystalpackingarrangementarecarriedoutinordertofine-tune thestructural andmacroscopicproperties, keepingthebalanceof thespatial requirementsandelectrostaticeffectsof thealteredmolecules inthecrystals, preservingtheirisostructurality.Evenso,thedefinitionofisostructuralityisnot explicitaboutseveral issues.Arethecorrespondingstructuresrequiredtohave the same stoichiometry, Z0, symmetryelements and the same spacegroup? Because it isnotobvious inthedefinition, studiesonstructureanalysisand softwarecalculatingvariousnumericaldescriptorsdevelopedforthequantitativecomparisonof thedegreeof similarityof isostructural crystalsself-define their criteria. The extent Read More …