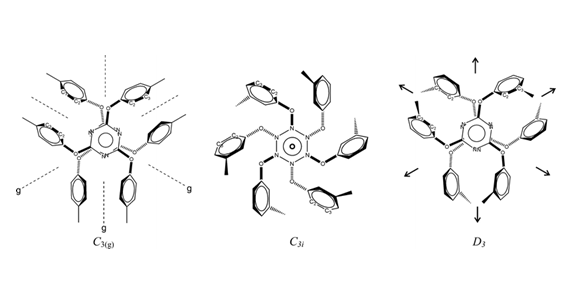
Petra Bombicz, Nikoletta B. Báthori, Alajos Kálmán Struct. Chem., 26, 1611-1619, 2015 Abstract 2,4,6-triaryloxy-1,3,5-triazines (POT) form in general molecular diads, termed as Piedfort Units (PU), in their crystals and their clathrates. Their bulky phenyl substituents (R = Me, F, Cl, Br, I, CN) in ortho, meta or para position substantially hinder internal rotations. Instead, non-crystallographic rotations (ncr) or translations (nct) are the bridges between the semirigid homologues or analogues, occasionally polymorphs. They occur in the space groups R3c (161), Read More …